Supercapacitor Material Activated carbon fiber (ACF) ,also known as fibrous activated carbon, is a kind of powder and granular activated carbon developed in the 1970s after the third generation of active functional materials. It is made of organic fiber by high temperature carbonization and activation. More than 50% of the carbon atoms are located on the inner and outer surfaces, forming a unique adsorption structure, which is called superficial solid. Due to its large specific surface area, rich micropore content, narrow pore size distribution, fast adsorption rate, small conductivity and thermal expansion coefficient and corrosion resistance, it is widely used in environmental protection, electronics, chemical and radiation protection, medicine and health, food and other fields.
1.1 Structure of activated carbon fiber-Supercapacitor Material
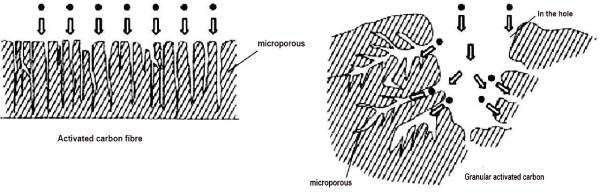
ACF is composed of microcrystals, surface heterocyclic or functional groups and very narrow pores. Microcrystal is a three-dimensional structure formed by carbon atoms stacked in the form of stone-like ink wafers, which has poor ordering and small size. The pore structure of ACF is different from that of activated carbon. As shown in Figure 2-4, more than 90% of the pores of ACF are micropores, which directly open on the fiber surface. The pore size is generally 1-4 nm, and the distribution is narrow, with almost no macropores and only a few mesopores. These micropores are produced after the removal of various carbides or disordered carbon between microcrystals in the preparation process. They are composed of fine capillary walls and are the main pore structures adsorbed. Adsorption superposition occurs between the relatively close microporous walls, causing the increase of the adsorption potential in the micropores. In addition, the micropores exposed on the surface can directly contact the adsorbent molecules, shorten the adsorption path, large driving force, fast adsorption speed, making the adsorption capacity of ACF large, high adsorption efficiency. ACF contains more than 90% carbon, and the rest is a small amount of hydrogen, oxygen and nitrogen, phosphorus, sulfur introduced by chemical activators.
🍎These non-carbon atoms combine with unsaturated carbon atoms on the ACF surface to form a unique surface structure. The types, distribution, polarity and acidity of the surface groups obtained are different with different activation methods. The surface structure of unsaturated carboxyl, carbonyl and phenolic groups, quinone, inside the oxygen containing groups such as ester base, amino and the amino sulfonic group and contains the functional groups, such as sulfur, nitrogen, halogen these functional groups on the one hand, the adsorption effect, on the other hand have REDOX function, for some chemical reactions can play a catalytic role.
1.2 Performance and characteristics of activated carbon fiber
1.2.1 Characteristics of activated carbon fiber
(1) the specific surface area is large, generally accessible, and the contact area with the adsorbent is large. (2) Microporous structure developed, pore volume, adsorption capacity is 1.5 ~ 10 times of ordinary granular activated carbon, so it has a very high adsorption efficiency. (3) The surface contains a large number of active functional groups, so that it has a strong REDOX ability, can play a catalytic role in some reactions.(4) Good molding, not easy powder, can be made into felt, cloth, paper, line and other forms, convenient for different uses and needs. (5) Acid resistance, alkali resistance, good electrical conductivity and heat resistance.
1.2.2 Performance of activated carbon fiber
🍐(1) Adsorption performance The structure of ACF is a non-polar adsorbent, and its specific surface area and pore size structure are the key factors affecting the adsorption performance. Compared with the general activated carbon, ACF has many excellent adsorption properties. ① Adsorption capacity of large ACF adsorption capacity can reach several times or even dozens of times of the traditional granular activated carbon, not only for inorganic, organic gas has a good adsorption capacityThe adsorption capacity of inorganic compounds, organic dyes and organophosphorus compounds in aqueous solution can also reach 5 times that of activated carbon. In addition, it also has a good adsorption effect on some microorganisms and bacteria, such as the adsorption rate of Escherichia coli up to 94% ~ 99%. ② Strong adsorption force in surface adsorption, the smaller the pore size, the larger the adsorption force field. Due to the narrow micropore of activated carbon fiber, its adsorption force field has a large holding effect, so that the adsorption capacity is significantly enhanced, so it is stronger than the ordinary adsorption material for the adsorption of low concentration substances, even for the order of 10-6 low concentration adsorbent still has a high adsorption capacity. ③ The adsorption speed is fast for the adsorption of gas can generally reach equilibrium in tens of seconds or minutes, and the adsorption of liquid only takes a few minutes to dozens of minutes to reach equilibrium. Similarly, because the fiber is thin, the outer surface is easy to be heated, so the desorption speed is also very fast.
(2) REDOX properties ACF surface has REDOX properties due to a series of oxygen-containing functional groupsThis property is manifested by its ability to reduce some metal ions with high electrode potential adsorbed from aqueous solution to zero or low valence and enrich on the surface of ACF. For example, the reduction in aqueous solution can be used for the enrichment, recovery and smelting of trace precious metals.
🍊(3) Conductivity ACF, like carbon fiber, has excellent conductivity and can be used as an electrode to degrade organic pollutants in wastewater. Because the enrichment of organic matter by ACF is beneficial to eliminate the concentration polarization effect, the electrolytic rate and degradation efficiency can be effectively improved. (4) Catalytic properties ACF also has gas-phase oxidation and catalytic reduction properties, which can reduce nitric oxide to nitrogen in the presence of ammonia gas. If the surface is loaded with other metal catalysts, the catalytic effect is more significant.
1.3 Preparation of activated carbon fiber
The main types of raw materials for ACF preparation are viscose, phenolic, asphalt, polyacrylonitrile, polyvinyl alcohol, polystyrene, lignin (coke wood) and natural plant fiber substrates (sisal, hemp, flax, jute), etc. Among them, the first four kinds of preparation technology are mature and have been large-scale production in industry. The characteristics of ACF prepared from various raw materials are shown in Table 2-2.
.jpg)
🍋Using different fiber materials, the specific conditions of ACF preparation are also different, but the basic process flow generally includes three main links: pretreatment, carbonization and activation.
1.3.1 Pretreatment
🍌Pretreatment includes salt or alkali impregnation and pre oxidation. Salt or alkali impregnation is to fully immerse raw fiber in salt or alkali (phosphate, sulfate, ammonium salt or alkali, etc.) solution, and then dry it. In the process of impregnation, salt or alkali molecules are immersed into the raw fiber, which can play a role in swelling, catalytic dehydration or cross-linking, preventing the fragmentation and escape of fiber molecules during heat treatment, thus improving the strength, yield and adsorption performance of activated carbon fibers. Pre oxidation is to place the raw fiber in an oxygen atmosphere and slowly pre oxidize it for a certain time or according to a certain heating program within a certain temperature range. After the raw fiber is pre oxidized, the linear polymer chain in the raw fiber will undergo oxidation, dehydrogenation, cyclization and other reactions to transform into a heat-resistant and stable trapezoidal structure, so that the fiber is not easy to melt and deform in the process of high-temperature carbonization, still maintains the shape of the fiber, and improves its yield after carbonization and activation.
1.3.2 Carbonization
🍉Carbonization is the most important link in the production of ACF. It mainly refers to the process of heating the raw fiber in an inert gas (such as nitrogen or argon) environment for a certain time, removing most of the non carbon components in the raw material, and rearranging the remaining carbon elements into carbon fibers similar to graphite microcrystalline structure by thermal condensation polymerization. After carbonization, the raw fiber becomes a carbon material with a certain mechanical strength and an initial pore structure suitable for activation, which plays a decisive role in the production of ACF, and also affects the subsequent activation reaction, thus directly affecting the structure and performance of ACF. The main influencing factors of carbonization process include carbonization temperature, heating rate, carbonization time, carbonization atmosphere and fiber tension control.
1.3.3 Activation
🍇Activation is to etch carbonized fibers with oxidizing gas at high temperature, and make ACF form developed microporous structure or expand pore diameter through surface treatment, thereby regulating its specific surface area and surface oxygen functional groups. The activation process is complex, but the basic principle is that the activated molecules react with the carbon atoms on the fibers to form rich micropores and surface oxygen-containing functional groups. The activation methods include physical activation, chemical activation, chemical physical joint activation, etc.
(1) Physical activation method
🍓The method mainly used in the pre industry uses oxygen, water vapor or carbon dioxide as the activator to etch and oxidize the disordered carbon part of the raw fiber into holes at a high temperature of 700~1000 ℃. Some studies suggest that physical activation can be divided into three basic processes: the gasification of non graphite carbon and heteroatoms; Reaction of graphite carbon; Reforming of graphite layer. First, the amorphous carbon blocking the hole is gasified and the hole is opened; Further activation, the carbon atoms on the surface lattice, dislocation and edge react at different rates to form gas leaving the surface, resulting in new holes; During deep activation, the pore is further widened
(2) Chemical activation method
🍈Chemical activation method is a method that uses potassium hydroxide, sulfuric acid, phosphoric acid, ammonia or zinc chloride and other chemical substances as the activator to make raw fibers contact with the activator through immersion and mixing and living reaction to form pores. Compared with the physical activation method, the activator in the chemical activation method can make the hydrogen and oxygen in the raw fiber escape mainly in the form of water vapor, inhibit the generation of by-product tar, thus increasing the yield of ACF and Its porosity and specific surface area. In addition, the chemical activation method can also reduce the carbonization and activation temperature of the raw fiber. However, chemical activation method is easy to cause environmental pollution and the product strength is poor.
(3) Chemical physical joint activation method
🍒Chemical physical joint activation method is an activation method that combines chemical activation with physical activation. Usually, chemical activation is carried out first and then physical activation is carried out. Because the two methods for preparing ACF are complementary in terms of process complexity, cost, and ability to control pore structure, the combination of the two methods can flexibly control the pore structure of ACF, and even prepare ACF containing only micropores or only mesopores. In addition, in addition to the activation method, the type of activator, activation temperature, activation time and concentration of activator are also key factors affecting the activation process. The structure and properties of ACF are directly affected by the activation conditions and degree.
1.4 Functionalization of activated carbon fiber
🍑The pore structure has a direct impact on the physical adsorption performance of ACF. Different pore structures will lead to different selective adsorption, adsorption capacity and adsorption rate. The surface oxygen-containing active functional groups of ACF will directly determine the catalytic performance. In order to give full play to the adsorption and catalytic properties of ACF, the pore structure, specific surface area and surface characteristics of ACF need to be adjusted and modified, that is, functionalization.
1.4.1 Aperture adjustment
🥭The pore structure adjustment is generally carried out in the carbonization and activation stages of ACF, including increasing the pore volume and specific surface area, increasing the proportion of micropores, and creating uniform pore size. The pore diameter of the carbon adsorbent and the molecular size of the adsorbate are adjusted to an appropriate proportion by adjusting the pore diameter to obtain the best adsorption effect. The commonly used adjustment methods are as follows: Due to the decomposition of hydrophilic oxygen-containing functional groupsACF has good hydrophobicity.
(1) Under the heating condition of carbon deposition method, ACF is in contact with hydrocarbon gas. As the carbon generated from hydrocarbon pyrolysis is deposited on the pore wall, the pore diameter is reduced. Therefore, proper pore diameter can be obtained by controlling the process conditions. The hydrocarbon organics used include methane, acetylene, isobutene, benzene, toluene and other hydrocarbons.
🍍(2) The metal compound catalytic activation method adds metal compounds to the ACF to increase the internal active points of the ACF micropores. When activated, the metal atoms selectively vaporize the highly crystalline carbon atoms, so that the micropores expand into mesopores. Generally, carbon atoms around metal atoms preferentially oxidize to form mesopores in fiber materials. ACF has good hydrophobicity.
(3) Under the heating condition of carbon deposition method, ACF is in contact with hydrocarbon gas. As the carbon generated from hydrocarbon pyrolysis is deposited on the pore wall, the pore diameter is reduced. Therefore, proper pore diameter can be obtained by controlling the process conditions. The hydrocarbon organics used include methane, acetylene, isobutene, benzene, toluene and other hydrocarbons.
🥝(4) The metal compound catalytic activation method adds metal compounds to the ACF to increase the internal active points of the ACF micropores. When activated, the metal atoms selectively vaporize the highly crystalline carbon atoms, so that the micropores expand into mesopores. Generally, carbon atoms around metal atoms preferentially oxidize to form mesopores in fiber materials.
In addition, there are also methods for adjusting the pore structure, such as evaporation and plating, catalytic activation of organic compounds, etc.
1.4.2 Surface modification
🎄(1) Oxidation method:The oxidation method mainly uses strong oxidants to oxidize the surface groups of ACF under appropriate conditions to increase the oxygen containing groups on its surfaceStrong surface polarity. There are three main methods of oxidative modification: gas phase method, liquid phase method and electrochemical method. The gas phase method is to increase the oxygen containing functional groups on the surface of ACF by reacting with oxidizing gases such as O2 or O3 at higher temperatures. Liquid phase method is to use strong oxidizing liquids (nitric acid, sulfuric acid, etc.) to react with ACF for oxidative modification, and nitric acid modification is the most studied. In electrochemical method, ACF is used as an electrode in the electrolyte to react with ions in the solution through its excellent adsorption performance and high surface catalytic oxidation performance. Basova et al. used ammonium persulfate as electrolyte solution. After electrochemical oxidation of polyacrylonitrile based ACF at 50 ℃, oxygen containing groups were produced on the surface of ACF, mainly including hydroxyl, carbonyl and carboxyl groups.
(2) The surface loading method is used to load metal compounds to adsorb oxidized metal ions (Au3+, Hg2+, etc.) on the surface of ACF, and then use the reducibility of ACF to reduce metal ions into simple or low valent ions, so as to increase the adsorption performance of ACF through the strong binding force of metal or ions on the adsorbate. Load removal In addition to the generic compounds, the surface of ACF can also be modified with organic compounds and inorganic molecules.
🌲(3)The plasma treatment method generates a large amount of plasma through corona discharge in the gas medium, and uses these high-energy plasma to impact the material surface, so as to change the physical and chemical properties of the material surface without damaging the material surface characteristics, thus improving the material specific surface area, pore diameter, pore volume, surface functional groups and other related properties.
(4)Microwave modification by microwave irradiation can decompose the oxygen-containing acidic groups (hydroxyl, carbonyl) on the surface of ACF in a short time, and introduce basic groups such as pyrrolidone on the surface of ACF to increase its surface pH value and enhance its chemical stability. This method is energy-saving, time-saving and efficient. In addition to the generic compounds, the surface of ACF can also be modified with organic compounds and inorganic molecules.
1.5 Application of activated carbon in supercapacitors
🌳Activated carbon is the earliest carbon electrode material used in supercapacitors. Due to its abundant raw materials, low price and high specific surface area, it is still the first choice for commercial supercapacitors. Since Beck proposed to use activated carbon as electrode of double-layer capacitor in 1954, the application of activated carbon in supercapacitor has attracted much attention. According to the different source of raw carbon, the application of activated carbon in supercapacitors is briefly introduced.
1.5.1 Phenolic activated carbon fiber
🌴Phenolic ACF has become an ideal electrode material for supercapacitors due to its high carbonization yield, large pore size, good conductivity and high strength.
In 1985, Panasonic Electric Company of Japan used phenolic resin activated carbon fiber with an average pore diameter of 2.5 nm for the preparation of electric plate materials for electric double-layer capacitors, which greatly improved the quality of the capacitors produced by the company. Zhang Yuqin et al. prepared phenolic ACF from phenolic fiber by KOH activation.
☘️The results show that 900 ℃ is the best temperature for KOH to activate phenolic fiber, and the sample has the best circulation, small internal resistance, specific surface area and specific capacitance. Although the products show different specific surface area and specific capacitance at different activation temperatures, their overall pore size distribution is basically the same. With the increase of activation temperature, the capacitive performance and power characteristics of the sample are improved,The internal resistance is reduced.
Yoshida et al. studied the relationship between the surface acidic functional groups of phenolic ACF in organic electrolyte and the electrochemical performance of electric double-layer capacitors. The research shows that acidic functional groups such as surface carboxyls, lipids and phenolic hydroxyl groups can cause leakage current of capacitors. Heat treatment under nitrogen at 1000 ℃ can effectively reduce the surface acidic oxygen-containing functional groups. Compared with asphalt and cellulose based ACF, phenolic based ACF after heat treatment has the lowest content of acidic oxygen-containing functional groups, the highest capacitance and the minimum leakage current.
1.5.2 Polyacrylonitrile based activated carbon fiber
🍀Xu et al. carbonized polyacrylonitrile based carbon fiber cloth at different temperatures, and then activated it with CO2 at 900 ℃ to obtain a series of ACF with different specific surface area and pore size distribution. It is found that the specific surface area and pore structure of ACF obtained at 600 ℃ carbonization are most suitable for supercapacitors, and the specific capacitance can still be obtained at the current density of, with high power characteristics. Li Ying took polyacrylonitrile as the precursorACF with large/mesoporous structure of in situ nitrogen rich hierarchical three-dimensional network was prepared by a new method combining wet spinning with KOH activation. This hierarchical pore ACF has high specific surface area, large pore volume and (mass fraction) high nitrogen atom content. Used in supercapacitors, it shows high energy density of high specific capacitance and excellent multiplying performance.
1.5.3 Asphalt based activated carbon fiber
🍃Li Haiyan prepared mesoporous ACF from general pitch carbon fiber by different activation processes, and compared the effects of cobalt salt immersion, primary activation and secondary activation on its specific surface area and pore structure. The results showed that the secondary activation energy increased the specific surface area and the mesopore ratio. Cobalt salt impregnation plays an obvious catalytic role in the activation process, but when the activation degree is too large, the addition of drilling salt will reduce the specific surface area and pore diameter of ACF.
1.5.4 Plant fiber based activated carbon fiber
🍁Liu Fengdan et al. used natural plant fiber ramie as raw material and ZnCl2 chemical activation method to prepare ACF at different activation temperatures. It was found that the specific surface area decreased with the increase of activation temperature. ACF supercapacitor activated at 650 ℃ has a specific capacitance of up to at constant current discharge, and has lower internal resistance, better power characteristics and longer cycle life.