Carbon nanotubes
Carbon nanotubes (CNT), also known as bucky tubes, belong to the Fullerian carbon system. In 1991, Professor Lijima discovered for the first time a nanometer-sized (1-100nm) multilayer tube, namely CNT, under a high-resolution transmission electron microscope when evaporating graphite electrodes using a vacuum arc. As a self-assembled monomolecular tubular material, CNT is considered to be an excellent supercapacitor electrode material due to its unique hollow structure and good electrical conductivity.
1 Structure of carbon nanotubes
CNT is a typical layered hollow structure, which can be regarded as a seamless nanoscale tube structure formed by curling single-layer or multi-layer graphite sheets around the same central axis at a certain helical angle, and the two ends are sealed by fullerene hemispheres. live, is a one-dimensional quantum material (as shown in Figure 2-7). Most of the carbon atoms in CNTs form bonds with the three surrounding carbon atoms in sp2 hybridization, forming a planar network structure with hexagons as the basic unit. In addition, some carbon atoms bond in sp3 hybridization to form Some pentagonal or heptagonal carbon rings.
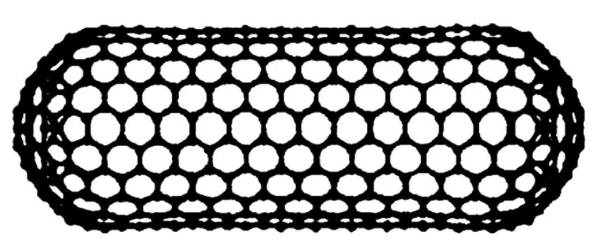
Figure 2-7 Structure of carbon nanotubes
According to the number of layers of graphite sheets in the tube, it can be divided into single-walled carbon nanotubes (SWNT) and multi-walled carbon nanotubes (MWNT). SWNT, also known as Fuller tube, is considered to be a single-layer graphene rolled up. Its diameter is generally 0.7-6nm, and its length is 1-50μm. There are few defects in the structure and it has higher uniformity (as shown in Figure 2-8. as shown in (a)]. MWNT is a tube formed by coaxially curling several to dozens of layers of graphite sheets. The number of layers varies from 2 to 50, and the interlayer spacing is (0.34±0.01) nm, which is comparable to that of graphite. The arrangement of the layers is disordered, usually the diameter of the multi-walled tube is 2-30nm, and the length is 0.1-50μm (as shown in Figure 2-8(b)).
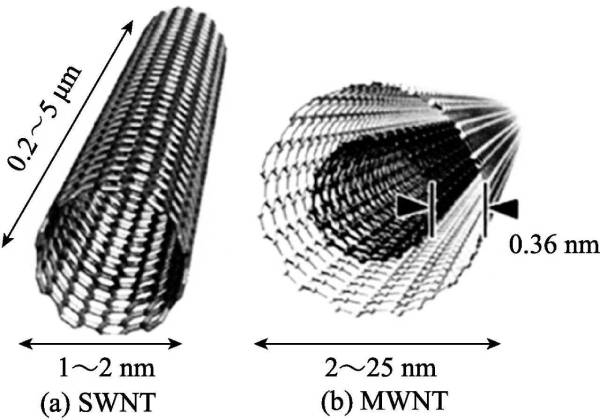
Figure 2-8 Structure of SWNT (a) and MWNT (b)
In the general CNT structure, the hexagonal grid of carbon atoms is helical, so the CNT has a certain degree of helicity. According to the helicity of graphite sheets constituting SWNTs, SWNTs can be classified into achiral symmetric type and chiral symmetric type. The mirror image of the achiral symmetric type is consistent with itself. According to the shape of the carbon ring in the cross section, the achiral SWNT is divided into two types: armchair type and zigzag type. The chiral symmetric type has a certain helicity, and its mirror image cannot coincide with itself, as shown in Figure 2-9.
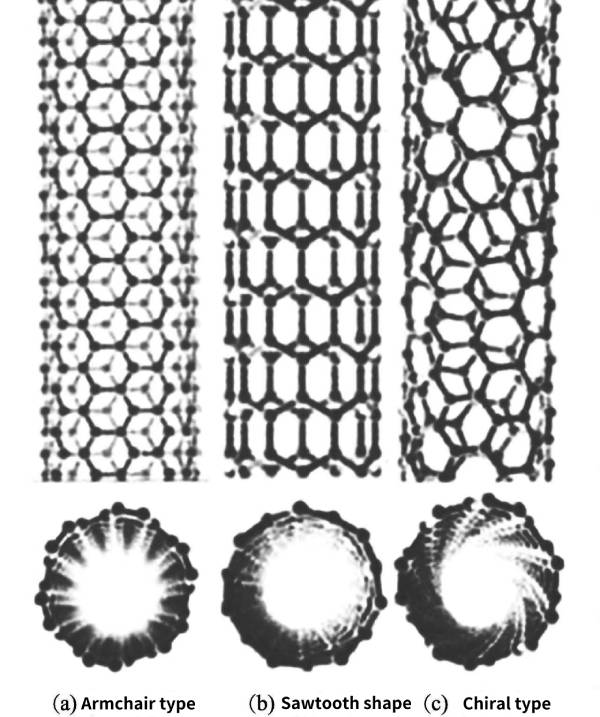
Figure 2-9 Three different types of carbon nanotubes
2 Properties of carbon nanotubes
CNT’s unique one-dimensional hollow tubular structure and huge aspect ratio make it have excellent mechanical, electrical, thermal, optical and other properties. Specifically in the following aspects.
2.1 Mechanical properties
The carbon atoms in CNT are sp2 hybridized, and the C C covalent bond formed is one of the strongest valence bonds in nature, which endows CNT with extremely high strength, toughness and elastic modulus. Its Young’s modulus and shear modulus are the same as those of diamond, and its theoretical strength can reach 1.8×1012Pa, which is 100 times that of steel, which is the highest modulus among known materials; its bending strength can reach 14.2GPa, and its stored strain energy can reach 100keV, which is twice that of the best micron whiskers; its elastic strain can reach 5% to 18%, about 60 times that of steel, and its density is only 1/6 of steel; CNT also has ultra-high toughness, It is estimated that its maximum elongation can reach 20%. The ratio of length to diameter of CNT can reach 100-1000, which is far beyond the aspect ratio of general materials (about 20). At the same time, CNT also has good bendability, and it will not produce brittleness even if it is subjected to a large external stress. These excellent properties make carbon nanotubes a “super fiber” material.
2.2 Electrical properties
Like graphite, CNT has a large number of p electrons, which can form a large range of delocalized π bonds, and unpaired electrons can move freely along the tube wall, but with different helix angles and tube diameters, its conductivity can be metallic or semiconductor sex. The tube diameter and helical structure are mainly determined by the chiral vector. When the chiral vector is an integer multiple of 3, CNT exhibits metal conductivity, otherwise it is semiconductor conductivity. Theoretical calculations show that when the tube diameter is less than 6nm, it mainly exhibits the metal performance of a good conductor, and when the tube diameter is larger than 6nm, its performance gradually turns into a semiconductor. It is reported that when the diameter of CNT reaches 0.7nm, it will exhibit superconducting properties
2.3 Thermal properties
CNT has a low coefficient of thermal expansion, ultrahigh thermal conductivity and thermodynamic stability. SWNT relies on ultrasonic waves to transmit heat energy, and the speed can reach 104m·s-1, which is one of the best heat-conducting materials in the world. When the temperature is 100K, the thermal conductivity of a single CNT is 37000W·m-1·K-1, and it can reach 6600W·m-1·K-1 at room temperature, and the thermal conductivity of MWNT is also over 3000W·m-1 ·K-1, and it will increase with the increase of temperature above 120K. CNT has good high-temperature stability, and the structure of small-diameter carbon nanotubes is still stable at a temperature of 3000K.
2.4 Optical properties
The optical performance of CNT presents strong nonlinearity, and the third-order nonlinear optical effect enables it to be used as an optical limiter with excellent performance and a high-speed all-optical switch. Under certain conditions, SWNTs can absorb photons and emit fluorescence in the near-infrared band. The optical polarization of CNT, especially the high degree of polarization in the ultraviolet region, has more application potential than other polarizing materials. The electroluminescence and photoluminescence of CNT have excellent properties such as high luminous efficiency, relatively constant resistance, and relatively stable structure. The luminescence of CNT is caused by the transition of electrons on two energy levels related to field emission. Studies have shown that the luminescence of SWNT occurs near the part supporting the nanotube, and the luminescence intensity increases with the increase of the emission current, while Light absorption decreases with increasing pressure.
3 Preparation of carbon nanotubes
Since Lijima prepared CNTs by the vacuum arc discharge method for the first time in 1991, it immediately attracted widespread attention of scientific researchers. How to prepare CNTs with high purity, few structural defects and uniform tube diameter is the basis of their performance and application research. At present, the commonly used CNT preparation methods mainly include: arc discharge method, chemical vapor deposition method, laser evaporation method, template method and so on.
3.1 Arc discharge method
The arc discharge method is the earliest and most typical method for preparing CNTs, which is mainly used in the production of SWNTs. The preparation principle of this method is to use pure graphite rods as electrodes, and in an inert gas atmosphere, discharge through the graphite electrodes to generate a high temperature above 3000 ° C, the plasma generated between the electrodes sublimates the carbon in the anode, and deposits on the graphite cathode. A product of CNTs. The arc discharge method is simple and fast, and the prepared CNTs have a high degree of graphitization, straight tube walls, good crystallinity, and a nearly perfect structure. However, due to the high arc temperature, the reaction is very violent, and it is difficult to control the process. In addition, this method has low yield, high energy consumption, and many by-products. The generated CNTs are mixed with C60 and other products, which is not conducive to subsequent purification and separation.
3.2 Laser evaporation method
The laser evaporation method is developed on the basis of the arc discharge method. The laser evaporation method uses graphite doped with transition metal catalysts such as Fe, Co, and Ni as the target material. At a reaction temperature of 1200°C, under the protection of an inert gas (He), the graphite target is bombarded with a laser to generate gaseous carbon, gaseous carbon and Catalyst ions are carried by the gas flow from the high temperature area to the low temperature area, and grow into carbon nanotubes under the action of the catalyst. The morphology of CNTs obtained by this method is similar to that obtained by the arc discharge method, but the purity and quality are higher, no amorphous carbon appears and continuous operation is allowed. The disadvantages of this method are high energy consumption, complicated experimental equipment, high preparation cost, and it is not suitable for large-scale production.
3.3 Chemical vapor deposition method
The chemical vapor deposition method, also known as the catalytic cracking method, is relatively mature in its preparation process and growth mechanism, and the method is simple and suitable for large-scale production of CNTs. This method mainly uses hydrocarbon compounds such as methane, ethylene, and benzene as carbon sources, and transition metals such as iron, cobalt, and nickel as catalysts. At a relatively low temperature of 500-1200°C, carbon source gas and When the catalysts are in contact with each other, they are cracked into carbon atom clusters on the surface of the catalyst particles and recombine to form CNTs.
Studies have shown that the diameter of metal catalyst particles largely determines the diameter of CNTs, so CNTs with higher purity and more uniform size distribution can be grown by selecting and controlling the catalyst type and particle size. The outstanding advantage of this method is that the residual reactant is gas, which can leave the system after the reaction to obtain relatively high-purity CNTs, and the reaction equipment is simple, low in cost, and large in output. It is the most likely effective method for large-scale industrial production of CNTs. . But its disadvantage is that the prepared CNT has a large number of defects, irregular shape, easy to bend and deform, and the degree of graphitization is low, and the stability is poor. Certain post-processing (such as high temperature annealing) can be taken to eliminate the defects and make the tube straight. enhanced.
3.4 Other methods
The template method uses a porous material with a pore size of nanometer to micrometer as a template, and combines electrochemical methods, precipitation methods, sol-gel methods, and gas phase precipitation methods to precipitate material atoms or ions on the pore walls of the inherent template. One way to get CNT.
The plasma method is to decompose the vapor of hydrocarbon compounds such as benzene through plasma to produce carbon atom clusters and deposit them on a cold metal plate to obtain ultra-long CNTs. In this method, CNT is grown in an epitaxial growth mode, and the growth rate can reach 0.1nm·s-1, but the method is complicated in equipment and expensive in cost.
The water/solvothermal method is a method in which carbon-rich substances are directly placed in a high-pressure reactor under hydrothermal or solvothermal conditions, and crystallized at a certain temperature to obtain CNTs. The method does not require high temperature and gas protection, which greatly reduces the preparation cost.
In the underwater arc method, two graphite electrodes are inserted into a vessel containing deionized water, and plasma is generated between the two electrodes through arc discharge, thereby depositing CNTs on the cathode. If inorganic salts are added to water, CNTs filled with metal and sealed at both ends can be obtained. The method has low reaction temperature and low energy consumption.
The in-situ growth method is to fully mix the polymer, functional fillers and catalyst precursors at a certain temperature, and then heat briefly at a temperature of 900 ° C to synthesize bundled CNTs in situ and with high yield without adding any catalyst. Methods. The obtained CNT has higher specific surface area and catalytic activity, and is a good catalyst support.
Solid-phase pyrolysis is a new method to grow CNTs by conventional solid-phase pyrolysis of carbon-containing metastable solids. This method has many advantages such as stable process, no need for catalyst, in-situ growth, etc., but due to the limitation of raw materials, its production cannot be scaled and continuous. So far, there are still many CNT preparation methods that have been discovered, but the only methods that can be used to prepare CNT in large quantities are graphite arc method and chemical vapor deposition method. Other methods have certain defects and cannot be used in industrial preparation.
4 Application of carbon nanotubes in supercapacitors
CNT has high crystallinity and good electrical conductivity. The size of the micropores can be controlled by the synthesis process. The unique mesoporous cavity structure can be intertwined to form a nanoscale network structure, which is conducive to the formation of an electric double layer. It is considered to be a Ideal electrode material for electric double layer capacitors.
4.1 Single-walled carbon nanotubes
SWNT has a higher theoretical specific surface area than MWNT, so it is expected to obtain higher specific capacity, but the preparation and purification of SWNT are more difficult, and the cost is much higher than that of MWNT. An et al. used the arc discharge method to synthesize SWNTs as electrode materials for supercapacitors, and discussed the influence of binder, carbonization temperature, discharge current density and other factors on their electrochemical performance. In this experiment, bundled SWNT with a purity of 20%-30% and 30% polyvinylidene chloride were mixed and pressed to form an electrode, heat-treated at 500-1000°C for 30min, nickel foil was used as a current collector, and 7.5mol L-1 KOH The electrolyte is assembled into a capacitor with a maximum specific capacity of 180F·g-1, a specific power density and an energy density of 20kW·kg-1 and 6.5W·h·kg-1, respectively. As the heat treatment temperature increases, the specific surface area of the electrode increases, the pore size distribution is improved, and the specific capacitance increases. An Yuliang et al. prepared SWNTs by chemical vapor deposition using coal gas as a carbon source, and used them as electrode materials for supercapacitors. The specific capacity was 55F·g-1 and the cycle performance was good.
4.2 Multi-walled carbon nanotubes
MWNTs usually exist in a discrete state, and the stacking pores between the tubes are all hollow, which is very conducive to the formation of an electric double layer. In addition, by adjusting the process parameters, the diameter of the inner cavity can be slightly larger than 2nm, so that the inner surface can also be used to form an electric double layer. Compared with SWNTs, MWNTs are more suitable as electrode materials for supercapacitors. Niu et al. made intertwined MWNTs from catalytic pyrolysis of hydrocarbons to make thin-film electrodes, which were used as supercapacitor electrode materials for the first time. In the H2SO4 electrolyte with a mass fraction of 38%, the specific capacitance reaches 49-113F·g-1, and the power density is greater than 8kW·kg-1. K. Jurewicz et al. mixed MWNT and KOH at a mass ratio of 4:1, and the specific capacitance of the CNT supercapacitor after high-temperature activation treatment reached 49F·g-1, which was 12 times that of the unactivated one. Further ammonia oxidation treatment, the specific capacity increased to 58F·g-1. This shows that after the activation and ammonia oxidation treatment of CNT, the functional groups on CNT increase, which is beneficial to improve the supercapacitive performance of CNT.
Frackowiaka et al. used acetylene catalytic cracking to prepare three different MWNTs with a specific surface area of 128-411 m2·g-1. The corresponding specific capacitance in 6mol·L-1 KOH is 4~80F·g-1. Using cobalt as a catalyst, the MWNT obtained by cracking acetylene at 700 °C was oxidized with concentrated nitric acid, and the specific capacity increased from 80 F·g-1 to 137 F·g-1, the specific surface area did not change much, but the cyclic voltammetry curve produced a significant The redox peaks indicate that the increase in specific capacity is due to the quasi-capacitance produced by the surface functional groups.
Wang Xiaofeng and others prepared MWNT materials by catalytic cracking method, and prepared electrodes with foamed nickel as the substrate. The inner core of the capacitor was made by superimposing 20 electrodes and non-woven diaphragms into the stainless steel inner shell, and injected 1mol L-1 LiClO4/PC organic electrolyte assembled into a supercapacitor. The capacitance of this capacitor can reach 600F at 20A current, the internal resistance is only 2.5mΩ, its specific power is 1kW·kg-1, and its specific energy is 0.8W·h·kg-1, even at 100A large current charge/discharge Under these conditions, the capacitance of the supercapacitor still reaches 570F.
4.3 Ordered carbon nanotubes
Freely grown CNTs have disordered orientations, different shapes, or aggregated into bundles, or intertwined with each other, and are even associated with amorphous carbon inclusions, making it difficult to purify, which affects their property research and practical applications. In recent years, highly ordered CNT arrays research has aroused people’s attention. Compared with ordinary CNTs, the vertically grown CNT arrays have regular pore structure and conductive pathways, which make them have higher effective specific surface area, lower ion diffusion resistance and better rate performance.
He Chunjian et al. used C2H2 as a carbon source to deposit ordered CNT arrays in porous alumina templates by chemical vapor deposition and made electrodes. The results show that the electroactive ions under the diffusion control potential are difficult to reach the depth of the inner wall of the CNT at a conventional diffusion rate, and most of the inner wall area is not fully utilized. Only a part of the inner wall near the tube mouth contributes to the diffusion, but its electric The capacity can reach 68.7mF·cm-2, indicating that the ordered CNT array electrode has excellent performance for supercapacitors.
Chen et al. used anodized aluminum as a template to prepare an ordered CNT array from C2H2 by chemical vapor deposition. It was observed that the tube diameter was uniform, the outer diameter was about 120nm, the wall thickness was only 5nm, and the length was about 0.26nm. Take a part as the working electrode, use 1mol L-1 H2SO4 as the electrolyte, under the current density of 210mA g-1, the measured specific capacitance is as high as 365F g-1, and the current density increases to 1.05A g-1 At 1, its specific capacitance is still as high as 306F·g-1, a decrease of only 16%, indicating that the electrode has good power characteristics. In addition, the ordered CNT array electrode also has low equivalent series internal resistance and good cycle stability.
4.4 Direct growth of carbon nanotubes on conductive substrates
Generally, the prepared CNT is in the form of powder or granules. To prepare it as an electrode of a supercapacitor, a certain amount of conductive agent, binder and slurry sheet must be added, and finally pressed together with the current collector, so that the CNT and the binder and the The contact resistance between the pole piece and the current collector becomes part of the internal resistance of the entire capacitor. If the CNT is grown directly on the conductive substrate during the preparation process, it can be used as an electrode directly without molding treatment, which will greatly reduce the contact resistance between the active material and the current collector, and simplify the preparation process of the electrode.
Chen et al. used acetylene as a carbon source and Ni as a catalyst to grow CNTs with uniform diameters on graphite sheets with a thickness of 0.067 mm by chemical vapor deposition. After soaking in 15% HNO3, they were used as electrodes for supercapacitors. In 1.0mol·L-1 H2SO4 electrolyte, at a scan rate of 100mV·s-1, the cyclic voltammetry curve still maintains a good rectangular shape, and its specific capacity reaches 115.7F·g-1.
Yoon et al. used 0.1nm-thick nickel foil as the substrate, NH3 plasma etching for 5min to make the surface rough and uneven, and used hot wire plasma-enhanced chemical vapor deposition to grow high-purity, oriented arrays with a thickness of about 20nm on it. Carbon nanotube arrays with a high degree of graphitization. Using 6mol L-1 KOH as the electrolyte and polypropylene film as the diaphragm to assemble a button capacitor, at a high scan rate of 1000mV s-1, the cyclic voltammetry curve still maintains a good rectangle, indicating that the directly grown carbon Nanotube electrodes have very low internal resistance and thus have high discharge efficiency and good power characteristics. The surface is further treated with NH3 plasma, the specific surface area is increased from 9.36m2·g-1 to 96.52m2·g-1, and the specific capacitance is also increased from 38.7F·g-1 to 207.3F·g-1.
Using ethylene as a carbon source and iron phthalocyanine as a catalyst precursor, Zhang Hao et al. used chemical vapor deposition to directly grow CNT arrays on the surface of tantalum sheets and stainless steel sheets, and used them as the positive and negative electrodes of supercapacitors. In the organic electrolyte of 1Et4NBF4/PC, the capacitor can obtain a working voltage up to 3.5V, and its specific power and specific energy performance are 928kW·kg-1 and 19W·h·kg-1, respectively.