✨Metal oxide (hydroxide) is currently the material with the highest specific capacitance and energy density. In addition, it has the advantages of rich raw materials, diverse morphology and structure, low resistance and high power density, and has become the recognized preferred material for supercapacitors electrodes.
🌟The supercapacitors with metal oxide as electrode material belongs to Faraday pseudocapacitor, also known as Faraday quasi capacitor. The electrode materials mainly include precious metal oxides and transition metal oxides. As the energy storage process of pseudo capacitor is realized through redox reaction, the surface and interior of electrode materials can participate in Faraday reaction, while the electric double-layer capacitor is connected to energy storage is realized by electrostatic adsorption on the surface of electrode material, so the specific capacitance of pseudo capacitor is much higher than that of double layer capacitor, reaching 10~100 times, which makes it possible to realize the miniaturization of supercapacitors.
⚡️The results show that the metal oxides suitable for supercapacitors electrodes generally need to have: ① conductive oxides; ② In a continuous range without phase change, there should be two or more coexisting oxidation states of the metal; ③ Its lattice should allow protons to be inserted freely (or removed by oxidation) through reduction to exchange O2 and OH -. So far, more and more metal oxides have been applied to pseudocapacitor supercapacitors, mainly including RuO2, MnO2, Co3O4, NiO, V2O5, Fe3O4, SnO2, Bi2O3, IrO2, etc.
1 Precious metal oxides
💥In the 1990s, when Conway was researching ruthenium oxide (RuO2) electrode materials, he found that it had the advantages of good conductivity, high stability, large specific capacitance, and fast charging and discharging. It was the first supercapacitors electrode to be researched. Material. After research, excellent electrochemical performance can be obtained, and its specific capacity is much higher than that of electric double layer capacitors. Among various oxides, RuO2 is considered to be the faraday pseudocapacitor electrode material with the best performance among the currently researched electrode materials for supercapacitors, and is considered to be the most promising material.
🌼As a supercapacitors electrode material, ruthenium oxide has many states, which can be generally divided into pure ruthenium oxide electrode material and composite ruthenium oxide electrode material. Pure ruthenium oxide electrode materials can be divided into crystalline materials and amorphous materials according to the form of ruthenium oxide. Since ruthenium oxide is expensive, it is a good choice to combine it with other materials with similar functions and cheap prices. Composite ruthenium oxide electrode materials can be divided into: composite materials with carbon, composite materials with other oxides, and composite materials with conductive polymers.
1.1 Crystalline ruthenium oxide electrode material and amorphous hydrated ruthenium oxide electrode material
☀️The U.S. Army Laboratory reported that the specific capacitance of its amorphous hydrate RuO2 can reach 768F per gram. Fang used thermal decomposition and oxidation method to make RuO2 thin film electrodes, and the specific capacitance of single electrode reached 380F; Hu prepared hydrated RuO2 by electrochemical deposition, the specific capacitance could reach 552F per gram, and the power density could reach 147kW per kilogram; Makino used liquid crystal material as template Electrodeposition of ordered mesoporous RuO2 materials, the specific capacitance can reach 12.6mF per square centimeter; Hu et al. successfully synthesized aqueous RuO2 (RuO2 xH2O) nanotube array electrodes using template synthesis and anode deposition techniques, with a maximum power density of 4320kW .J.P. Zheng Preparation of amorphous RuO2 xH2O electrode material by low-temperature annealing by sol-gel method.
🌈In its bulk phase, H+ is easily transported, so redox reactions can be carried out not only on its surface but also in its bulk phase. The electrode material has a high utilization rate, a specific capacitance of 768F, and an energy density of 96J; B. O. Park et al. obtained a thin-film RuO2 electrode by cathodic electrodeposition. Studies have shown that the specific capacity and charge-discharge interval are determined by the thickness of the electrodeposited RuO2 film. When its mass density is 0.0014g, the specific capacitance of the electrode reaches 788F. But when the thickness of RuO2 film continues to increase, its surface morphology changes, the porosity of the outer layer decreases, and a dense inner layer is formed. On the contrary, its specific capacity decreases. Xia et al. RuO2 was prepared by hydrothermal reaction using RuCl3 as precursor. The results show that the material has high energy density and specific capacity, and good cycle stability. After 2000 cycles, its capacity value remained at 97% of the original value.
💐Highly dispersed nanoscale RuO2 particles have high specific capacitance, high energy density, high power density and good stability. Figure 3-1 shows the different morphologies of RuO2 in supercapacitors. Sho Makino et al. Japan studied the capacitive properties of low crystal water RuO2 nanoparticles and high crystal water RuO2 nanosheet electrode materials in the bioelectrolyte Acetobacter buffer. The experimental results show that the specific capacitance of RuO2 nanosheets in 5mol Acetobacter buffer reaches 1038F. The research results provide a new idea for the development of environmentally friendly materials.
1.2 Ruthenium dioxide/carbon composite electrode material
🌷In recent years, various RuO2/carbon composite electrode materials have also attracted attention. Carbon materials used as electrodes for electrochemical supercapacitors mainly include activated carbon powder, carbon black, carbon fiber, glassy carbon, carbon aerosol, carbon nanotubes, etc. Depositing RuO on high-surface-area carbon materials by various methods can increase the specific surface area of RuO, thereby increasing the specific capacitance of the material; adding ruthenium oxide to the carbon material can increase the specific capacity of the carbon material. For example, the specific capacitance of RuO2/multi-walled carbon nanotube electrodes in sulfuric acid aqueous solution is as high as 1652F. The conductive carbon matrix greatly enhances the conductivity of the material, facilitates the penetration of ions, and significantly shortens the ion transmission distance. Wu used the sol-gel method and low-temperature heat treatment techniques to load RuO2 onto the graphene surface. Due to the high specific surface area and high conductivity of graphene, the utilization of RuO2 active material is greatly improved. It was found that when the loading amount of RuO2 reached 38.2%, the specific capacitance of the composite material was about 570F, which was similar to that of pure RuO2, and the composite material could significantly reduce the preparation cost of the electrode.
1.3 RuO2/conductive polymer composite electrode material
🌹With the broadening of research scope, RuO2/polymer composites have also received more and more attention, including RuO2/polyaniline, RuO2/polypyrrole and RuO2/polyethylene (3,4-ethylenedioxythiophene). Lin Zhidong et al. prepared the nanocomposite of RuO2 and polyaniline (PANi) by in-situ polymerization. When the RuO2 content is 3%, the specific capacity of the composite electrode reaches 373.27F. S. Cosnier et al compounded polypyrrole with RuO2 in different proportions to obtain higher specific capacity, but the power density needs to be improved
1.4 RuO2/other oxide composite electrode materials
🌺It is reported that ruthenium oxide and manganese oxide, nickel oxide, lead oxide, tungsten oxide, indium oxide, tin oxide, strontium oxide, cobalt oxide, tantalum oxide and other oxide materials with pseudo capacitance effect can be obtained by coprecipitation, colloid method, sol-gel method and other methods. The specific surface area of these binary metal oxide composite electrodes increases with the introduction of mixed metal oxides. At the same time, the use of these cheap materials can reduce the preparation cost of the electrodes, and of course, the functions will also decrease. However, the overall performance of the materials (such as strontium oxide and indium oxide) will also be improved due to the synergistic effect of electrochemical performance. In addition, oxides without pseudocapacitance effect, such as silicon oxide and titanium oxide, are also made into various carriers with high specific surface area (such as film, mesh, nanotube and aerogel), and then ruthenium oxide is deposited on the carriers to increase their specific surface area and specific capacity. Yokoshima et al. successively prepared RuO2 and MoOx, VOx, TiO2, SnO2 and other composite electrode materials by the sol gel method, which played an excellent electrochemical performance of the composite electrode materials while reducing the amount of RuO2. Pani et al. coated Ru (OH) 4 and Ti (OH) 4 sol formed by hydrolysis of RuCl3 and TiCl3 in hydrochloric acid solution on Ti substrate to prepare Ti/TiO2+RuO2 composite electrode materials.
🌸At present, RuO2 materials are mainly used in aerospace, military science and other fields. Due to limited ruthenium resources and high prices, large-scale application is difficult. In addition, among the noble metal oxide electrode materials, IrO2 or RhOx materials are used as electrodes, which have Faraday pseudocapacitance characteristics similar to RuO2 electrodes. They all have good conductivity and can obtain high specific capacity and specific energy, but they are expensive. Therefore, it is necessary to find alternative materials or add other materials to reduce their consumption.
2 Transition metal oxides/hydroxides
🌼Although precious metal oxides and their composites can provide very high specific capacitance in supercapacitors, their high cost greatly limits their application in commercial production. Therefore, researchers are trying to explore using other transition metal oxides/hydroxides to replace noble metal oxides as electrode materials. Some cheap metal oxides, such as NiO, MnO2, Co3O4, SnO2, V2O5, have similar properties with RuO2, and are rich in resources and cheap in price, which has attracted extensive attention from researchers at home and abroad. Therefore, it has super capacitance characteristics.
2.1 Nickel oxide
🌻Nickel electrode materials have high specific capacitance, good magnification performance and stability, and are rich in reserves, low in price, green and non-toxic. It is a class of electrode materials with great development potential. Nickel oxide is mainly used in supercapacitors in the form of thin films. Its preparation methods include chemical precipitation, sol gel method, electrochemical method and template method. In addition, various nano nickel oxides with different morphologies, such as nanowires, nanoribbons, porous membranes, nanocones, nanorods, nano layers and nanospheres, have also been tried to be used as electrode materials for supercapacitor.
🌞Wang et al.obtained nickel oxide nanoribbons by simple hydrothermal method. Under 5A current density, its specific capacitance can be as high as 600F,The specific capacitance retention rate is 95% after 2000 cycles. Deng Meigen et al. prepared Ni (OH) 2 ultrafine powder by precipitation conversion method, and obtained NiO with an average diameter of about 10nm by heat treatment at 300 ℃. This method has simple experimental equipment and easy control of process conditions.
🌝The obtained nano NiO supercapacitors has typical Faraday pseudocapacitance characteristics. Under the charge/discharge current density of 2mA · cm-2, the specific capacity of electrode material reaches 243F.In spite of this, the reported specific capacitance of nickel oxide is still lower than its theoretical value, which can be attributed to its poor cycling performance and low conductivity.
🌛In order to solve these problems, it is an effective method to combine nickel oxide with cobalt oxide or conductive carbon materials to form composite materials. Because of the highly active nickel oxide surface layer, this composite material shows very high specific capacitance (900F), energy density (60W) and power density (10W); Symmetrical supercapacitors assembled from graphene/nickel oxide composites can also produce high specific capacitance (220F); In another work, nickel oxide repairThe specific capacitance of the composite was 816F · g-1 and its magnification property was good after three-dimensional graphene was decorated; The composite of nickel oxide and carbon nanotubes can improve the utilization of active substances in the materials and the specific capacitance can reach 384F · g-1.
🌕Liang Kun et al.constructed high-performance supercapacitors electrode materials such as nano porous NiO film and LaNiO3 doped mesoporous NiO film based on nano porous Ni film. These materials have large specific surface area, reasonable pore size distribution, and good conductivity. They can be directly used as electrode materials without using organic binders, showing good electrochemical performance. Although nickel oxide and nickel hydroxide have high theoretical specific capacitance and low cost, their potential windows are relatively low. How to increase the potential windows to meet the needs of practical commercial applications is still a problem to be solved.
2.2 Cobalt oxide and cobalt hydroxide
🌖Among various metal oxides, Co3O4 has been regarded as an advanced material with potential to replace RuO2 due to its low cost, high redox activity, theoretical specific capacitance up to 3560F, good reversibility and environmental friendliness.
🌱Lin prepared ultrafine Co2O3 electrode active material by alkoxide hydrolysis, and the specific capacity of single electrode reached 246F. However, CoOx dry gel synthesized by alkoxide sol gel method can obtain the maximum specific capacity of 291F at 150 ℃, which is very close to the theoretical value of 335F, and the cycle performance is stable.
🌿In recent years, scientists have also been working on the synthesis of Co3O4 nanostructures with different morphologies, such as nano layers, nanowires, nanotubes, nanorods, gel and microspheres . The specific capacitance of Co3O4 nano layer array can reach 2735F. Due to its unique three-dimensional layered structure, it has fast ion and electron transport characteristics. Mesoporous Co3O4 nanowire array can achieve a specific capacitance of 1160F, coated on foam nickelThereafter, the retention rate was 90.4% . Cobalt oxide nanotubes also exhibit good capacitive properties due to their unique structure and large specific surface area
☘️In order to improve the conductivity of Co3O4 electrode, it was compounded with various carbon materials and applied in supercapacitors. For example, compared with pure Co3O4, the specific capacitance of cobalt oxide/carbon nanotube composite synthesized by coprecipitation method is significantly increased to 418F, which is due to the synergistic effect between Co3O4 and carbon nanotube. The maximum specific capacitance of the aqueous solution of graphene/Co3O4 composites is 243.2F. Three dimensional graphene foam based Co3O4 nanowires have a specific capacitance of 1100F and excellent cycle stability. The flexible Co3O4/graphene/carbon nanotube paper composite electrode shows a specific capacitance of 378F.
🍀The specific capacitance produced by potential deposition of stainless steel on cobalt hydroxide nano layer is 890F. Porous cobalt hydroxide/nickelThe conductivity of the composite was improved due to the introduction of nickel, and the specific capacitance was 1310F. The sea urchin like mesoporous cobalt hydroxide nanowires show a specific capacitance of 421F. The higher specific capacitance of the material is attributed to its ordered structure, layered porosity and good electrical conductivity. In order to further improve its electrochemical performance, conductive carbon materials (such as carbon nanotubes and graphene) have been used to construct cobalt hydroxide composite nanostructures . The cohesionless cobalt hydroxide/carbon nanotube array electrode produces high capacitance (12.74F) and has excellent high magnification performance . Compared with pure cobalt hydroxide (726.1F), graphene/cobalt hydroxide composite exhibits high specific capacitance (972.5F). Although cobalt hydroxide and its derived composites show high specific capacitance, their practical application in supercapacitors will be greatly limited due to the low content of active substances and narrow potential range.
2.3 Manganese oxide
🍄Manganese based metal oxides have become the research focus of electrode materials for supercapacitors due to their low price, extensive resources, environmental protection and excellent electrochemical performance. At present, various MnO2 with different crystal structure, morphology and particle size have been studied, synthesized and applied to supercapacitor, and its theoretical specific capacitance can be as high as 1370F. There are many synthesis methods of MnO2 as electrode material of supercapacitor, such as low-temperature reduction method, chemical coprecipitation method, hydrothermal solvothermal synthesis, chemical microemulsion method, sol gel method, solution combustion method, solid phase reaction method, microwave assisted synthesis and deposition method.、
🌴Dangerous earthquake Kun prepared α、β、δ、γ The results show that they all have good capacitive characteristics, α- MnO2 has the highest specific surface area and porosity, its specific capacitance is up to 136F · g-1, but its high current discharge performance is poor, and the other three kinds of MnO2 have the same specific surface area. β- Although the specific capacitance of MnO2 is low (117.1F), its reasonable pore structure makes it have the best magnification characteristics and cycle stability.
🌳M.Kundu et al. Li Wenyao prepared single crystals by hydrothermal synthesis α- MnO2 ultra long nanowires, synthesized with surfactant α- MnO2 nanowires and nanorods. Through comparison, it was found that the electrochemical performance of the super long nanowire MnO2 electrode was the best, and its specific capacitance could reach 345F · g-1 when the current density was 1A · g-1. Wang et al. used one-step hydrothermal method to synthesize foam nickel loaded mesoporous MnO2/Ni (OH) 2 nanosheets. With the help of foam nickel high conductive framework, its specific capacitance reached 843F · g-1 when the current density was 2A · g-1. These indicate that the electrochemical performance of the electrode can be effectively improved by synthesizing nano materials with special morphology and structure.
🌲Yang et al. The charge/discharge test results show that the specific capacitance of the electrode material reaches 204F, and its capacity retention rate is close to 100% after 1000 cycles. Dong et al. obtained MnO2 nanoparticles through in-situ redox reaction between permanganate ions and carbon and embedded them in the mesoporous wall of carbon materials. This new structure of MnO2/mesoporous carbon composite has a specific capacitance of more than 2000F, and has high electrochemical stability and high reversibility.
🎄MnO2 has poor conductivity, so other metal elements (Co, Ni, Al, Mo, V, Fe, etc.) are mixed with MnO2 to form mixed oxides, and the conductivity of MnO2 is increased by introducing more carriers .
🌵Proper doping can also effectively prevent MnO2 from dissolving, thus improving the electrochemical reversibility and cycle stability. Vanadium doping can effectively inhibit the growth of MnO2 crystal, while iron doping can improve the crystallinity and structural stability and reduce the concentration of unstable Mn3+[37]. The incorporation of metal oxides such as SnO2 and Co3O4 can provide a fast path for electron transport and increase the electrochemical applications of amorphous MnO2.
2.4 Iron oxide
🐲Iron oxides FexOy, such as Fe3O4 and Fe2O3, have low cost, relatively high theoretical Faraday capacitance and high conductivity (Fe3O4 is about 2 × 104S · m) and environmental friendly, but its electrochemical performance needs to be improved due to poor conductivity. It has been reported that the specific capacitance of hydrothermally synthesized iron oxide is 340.5F, and its electrochemical performance strongly depends on the grain size.
🐉In order to improve the electrochemical performance of iron oxide, many researchers have constructed nanostructures or carbon materials or conductive polymers with conductive phases. For example, the specific capacitance of graphene/iron oxide composite nanotubes is 215F, which is 7 times that of pure Fe2O3 [38]. Jin Yuhong et al. [39] prepared Fe2O3 hollow shuttle nanoparticles by template free hydrothermal method. The hollow pore structure can limit the electrolyte. While increasing the reaction site of the active substance, it shortens the transmission distance between the active substance and the electrolyte, ensuring the rapid transmission of ions. When the current density is 0.5A · g, the specific capacitance of the electrode material is 249F. Highly ordered α- Due to its unique nanostructure, iron oxide nanotube arrays can provide a fast transportation route and a solid structure, with a specific capacitance of up to 138F · g-1. The maximum specific capacitance of graphene/ferric oxide/polyaniline ternary composite is 638F · g-1. In potassium hydroxide solution, its power density is 351W, energy density is 107W, and the specific capacitance attenuation is only 8% after 5000 cycles. The specific capacitance of carbon nanotube/nano-Fe3O4 composite is 165F, and after 1000 cycles, the specific capacitance is still about 85%.
🦄Recently, some researchers found that the external magnetic field has a great influence on the properties of graphene/iron oxide composites, and has obvious capacitance enhancement ability. At present, the electrochemical properties of iron oxides have not fully met expectations. Low conductivity and poor cycle stability are still the problems in the application of iron oxides in supercapacitors electrode materials.
3 Metal oxide composite
🦉As a new electrode material for supercapacitors, composite materials have become a research hotspot in recent years. Although metal oxide supercapacitors have developed rapidly and made some progress in theory and practice, the electrochemical properties of metal oxide supercapacitors still need to be further improved. The performance of supercapacitors can be effectively improved by compounding metal oxides with other materials with excellent capacitive properties and utilizing the synergistic effect between components.
3.1 Composite materials of different metal oxides
🐭Tao Jiayou et al. used MnO2 and molybdenum trioxide with the largest work function difference in transition metal oxides as the positive and negative electrode materials of asymmetric supercapacitors. MnO2 nanowires and molybdenum trioxide nanoribbons were synthesized by hydrothermal method, and carbon nanotubes were doped in a certain proportion to improve the conductivity of the electrodes. The results show that the operating voltage window of the asymmetric supercapacitor composed of these two electrodes is 0 ~ 2.0V, and its volume specific capacitance is 50.2F at the scanning speed of 2mV · s-1. After further inserting an intermediate layer with an inline structure between the positive and negative electrodes, the operating voltage window of the device can reach 0-4.0V. When the power density is 261.4mW, the energy density of the capacitor can reach 28.6mW. After 10000 cycles of testing, the device can retain 99.6% of the initial capacitance. Xu et al. prepared flower like NiO/Co3O4 metal oxide composites by hydrothermal method, andThe Ni/Co ratio in the reaction solution regulates the morphology and electrochemical properties of the metal oxide composites.
🍏In 2017, Zheng Xin designed an energy band structure conducive to interface electronic transmission through the composite of different metal materials. TiO2 embedded layer was introduced at the interface of ZnO/Ni (OH) 2, and ZnO/TiO2/Ni (OH) 2 core-shell structure nanowire arrays were prepared as cathode materials, which effectively reduced the electronic interface transport barrier during charging, reduced the activation energy in reduction reaction, and increased the capacitance. He further prepared ZnO/Fe2O3 composite nano materials by hydrothermal method as the cathode of the supercapacitors, assembled an asymmetric supercapacitors, and expanded the voltage window of the device to 1.6V. When the current density is 1A, the specific capacitance is l46.8F · g-1, and when the power density is 1350W, the energy density is 52.22W. Later, he further optimized the interface electron transport performance of cathode materials, and used hydrothermal method to grow ZnO/NiO core-shell structure nanowire arrays on three-dimensional carbon cloth substrate as flexible self-supporting electrodesGold nanoparticles are embedded at the interface of ZnO/NiO by ultraviolet reduction. When the current density is 5A, the capacitance of ZnO/Au/NiO electrode material is 4.1F, which is 720% higher than that of ZnO/NiO electrode material (0.5F). The embedding of Au can form a potential well between ZnO and NiO. During the charging process, a small amount of electrons can be captured, further improving the charge storage capacity of electrode material.
3.2 Carbon/metal oxide composite-Supercapacitor
🍎Carbon/metal oxide composites are widely used as electrode materials in supercapacitors, including carbon nanotubes (CNT)/metal oxide composites and graphene/metal oxide composites.
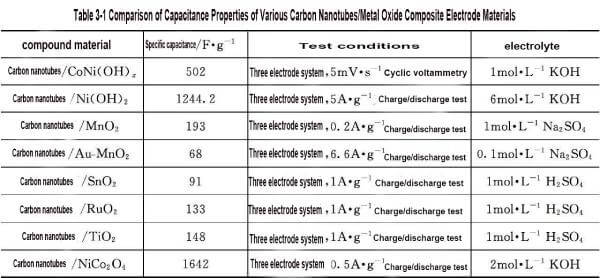
🍐Chen et al. prepared CNT/Ni (OH) 2 composite by direct hydrothermal method. This preparation method makes CNT uniformly dispersed in Ni (OH) 2 particles. Using 6mol solution as electrolyte, when the charging current is 5A, the specific capacitance of the composite is 1244.2F; When the current density is 20A · g-1, the specific capacitance remains at 771.3F · g-1, which is significantly higher than Ni (OH) 2 particles (372.1F) and CNT (101.4F). Based on the high specific surface area and high chemical stability of carbon nanotubes, the preparation of carbon nanotubes/metal oxide composites as support materials has been rapidly developed. However, due to different types and composite methods of oxides, the specific capacities of carbon nanotubes/metal oxide composite electrode materials vary greatly, as shown in Table 3-1.
🍊Graphene/metal oxide (hydroxide) composite materials use graphene oxide as the carrier, and use the oxygen containing groups on its surface as the anchor points of nanoparticles to deposit metal oxide particles on its surface. The synergistic effect of the two materials not only maintains the specific power of the supercapacitors, but also increases the specific energy and cycle stability of the system, and obtains electrode materials with excellent comprehensive performance. Chen et al. used the electrochemical deposition method to in-situ grow flower like MnO2 nanoparticles on the graphene surface, and the MnO2 nanoflowers uniformly grew on the graphene sheet to form a uniform electrode structure.
🍌The existence of MnO2 nanoparticles can increase the distance between graphene sheets, thereby promoting ion diffusion. Asymmetric supercapacitors is assembled with graphene as anode and MnO2 coated graphene as cathode. The specific capacitance and specific power of the capacitor are 328F · g-1 and 25.8kW · kg-1 respectively. Lee et al. used hydrothermal synthesis method to load Mn3O4 nanoneedles on graphene. In 1mol · L-1 Na2SO4 electrolyte, when the charge/discharge current density is 0.5A · g-1, 1A · g-1, 2A · g-1, 5A · g-1, 10A·At g-1, 15A · g-1 and 20A · g-1, the specific mass capacitances of the composites were 121F · g-1, 115F · g-1, 107F · g-1, 97F · g-1, 88F · g-1, 85F · g-1 and 83F · g-1 respectively (potential window was – 0.1~0.7V). After 10000 charge/discharge cycles (5A · g-1), the capacity retention rate was close to 100%.
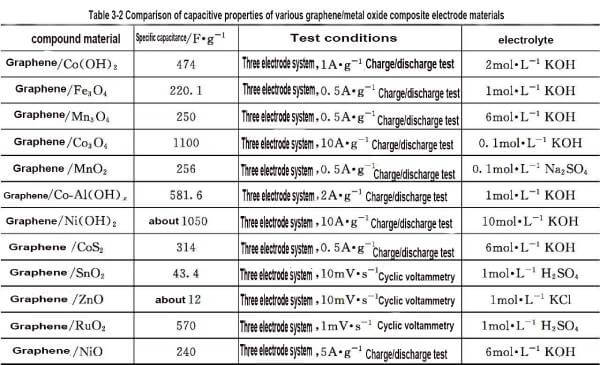
🍉Compared with nano Mn3O4 electrode, it is found that the specific capacitance of graphene/Mn3O4 composite material is significantly increased, indicating that with the conductivity of graphene, the internal resistance of electrode material can be reduced and the effect of electrochemical polarization can be reduced; And the high specific surface area of graphene also improves the utilization rate of Mn3O4, thus increasing the specific capacitance of the composite. Similarly, the specific capacity of different graphene/metal oxide composite electrode materials varies greatly, as shown in Table 3-2.
🍋Although the composite of metal oxide particles and graphene can effectively improve the capacitance, and the composite is simple and easy, and the material structure and performance are diverse, there are also shortcomings such as high metal oxide price and heavy metal pollution.