1 Historical development of supercapacitor
Supercapacitor first appeared in the middle of the 18th century. Pieter Van Musschenbrock of Leiden University in the Netherlands and Ewald Geory Von Kleist of Germany developed the Leyden jar, which is recognized as the prototype of all capacitors.
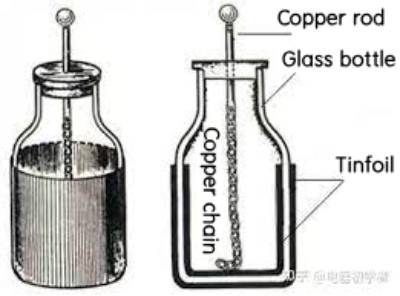
Supercapacitor predecessor
In 1879, Hermann Ludwig Ferdinand Von Helmholtz discovered the interface double electric layer phenomenon.
In 1957, Becker obtained the patent of electric double layer capacitor, which made a new breakthrough in the production of capacitors.
In the late 1960s, the appearance of a super capacitor made the research and application of capacitors develop rapidly. The research on supercapacitors mainly focuses on the development of novel electrode materials, the selection of appropriate electrolyte, and the optimization of capacitor assembly technology.
Supercapacitors, also known as electrochemical capacitors, gold capacitors, or Faraday capacitors, are energy storage devices that bridge the gap between traditional batteries and conventional capacitors. They offer unique advantages and characteristics that make them suitable for specific applications. Here are some key features and benefits of supercapacitors:
- Electrostatic Energy Storage: Supercapacitors store and release electric energy through a purely physical process, established by an electrostatic field. Unlike batteries, they do not involve chemical reactions during charging and discharging.
- Long Service Life: Supercapacitors have a longer service life compared to many other energy storage technologies. The electrodes and electrolyte in supercapacitors do not undergo chemical changes over time, contributing to their durability and reliability.
- Fast Charging and Discharging: Supercapacitors can charge and discharge rapidly. They can handle high current levels and are suitable for applications that require quick energy bursts, such as regenerative braking in electric vehicles or power backup systems.
- High Charge Storage Capacity: Supercapacitors have a significantly higher charge storage capacity compared to ordinary capacitors. This is typically several orders of magnitude higher, making them well-suited for applications that require higher energy density than traditional capacitors can provide.
- Energy Storage and Release: Supercapacitors are capable of efficiently storing and releasing energy in a controlled manner. This makes them suitable for applications that require energy smoothing, load leveling, and short-term energy storage.
Supercapacitors find applications in various fields, including renewable energy systems, transportation (e.g., hybrid and electric vehicles), consumer electronics, industrial machinery, and backup power systems. While supercapacitors offer several advantages, they also have limitations, such as lower energy density compared to batteries, which means they may not be the best choice for all energy storage applications. The choice between supercapacitors and batteries depends on the specific requirements of the application and the trade-offs between energy density, power density, and cycle life.
At present, electrode materials can be divided into three categories: carbon materials, transition metal oxides and conductive polymer materials.
2 Definition and characteristics of supercapacitor
2.1 Definition of supercapacitor
Supercapacitor, also known as electrochemical capacitor (EC), gold capacitor and farad capacitor, is a new energy storage device between battery and flat capacitor. Unlike batteries, supercapacitors do not undergo chemical reactions during charging/discharging. The storage or release of electric energy is a physical process established by an electrostatic field. The electrodes and electrolyte almost do not age, so they have a long service life,It can realize fast charging and large current discharge. In addition, its charge storage capacity is nearly 3-4 orders of magnitude higher than that of ordinary capacitors, so it is called “super” capacitor.
2.2 Characteristics of supercapacitor
As a new green energy storage device between traditional capacitors and rechargeable batteries, supercapacitors have the following performance advantages:
(1)Ultra high electrical capacity (0.1~6000F). The capacitance of traditional capacitors is small, while the capacitance of super capacitors is up to 6000F, thousands of times that of tantalum, aluminum and other electrolytic capacitors, which can meet the operation requirements of complex equipment.
(2)High power density. The output power density of the supercapacitors can reach 10kW · kg-1, hundreds of times that of the chemical battery. It can emit hundreds to thousands of amperes of current in a very short time, and can be used for high power output equipment.
(3)Fast charging speed. As the charging/discharging of supercapacitors is a physical process or a fast reversible chemical process on the electrode surface, it can be charged with large current and completed in tens of seconds to several minutes to achieve a real sense of fast charging.
(4)Ultra long cycle life. The super capacitor is based on the highly reversible adsorption/desorption mechanism of ions. It is not easy to have the phenomena that affect the service life such as the crystal transformation and shedding of active substances. The theoretical cycle life of carbon based capacitors is infinite, which can actually reach more than 100000 times, 100 times higher than that of chemical batteries.
(5)Wide temperature range. The reversible adsorption/desorption process of electrode material surface of supercapacitors is little affected by temperature, so it can be used in a wide temperature range (- 40~+70 ℃).
(6)High charging/discharging efficiency. Due to its high reversibility, the charge/discharge efficiency of supercapacitors can reach 98%, which is obviously superior to that of chemical battery.
(7)Light weight, maintenance free, less pollution, safety and environmental protection.
3 Composition of supercapacitor
3.1 Electrode materials for supercapacitor
Electrode is the core component of supercapacitors, which mainly generates double electric layers and accumulates charges. Therefore, electrode materials should have large specific surface area, no reaction with electrolyte, good conductivity and other performance characteristics. Carbon materials, metal oxides and conductive polymers are common.
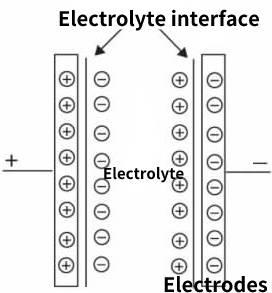
Working principle of supercapacitor
3.1.1 Carbon electrode materials
Carbon material is the most commonly used electrode material for supercapacitors, and it is also the electrode material that has been commercialized successfully at present. Carbon materials have high specific surface area and good electronic conductivity. In addition, they are rich in content, low in cost, easy to process, non-toxic, and have high chemical stability. At present, the commonly used carbon materials mainly include activated carbon, carbon aerogel, carbon nanotubes and graphene.
3.1.2 Metal oxide electrode materials
In general, transition metal oxides have higher energy density than traditional carbon materials and more stable electrochemical properties than conductive polymers. It can not only produce double electric layers like carbon materials to store charges, but also generate pseudo capacitance by Faraday reaction with electrolyte ions. At present, the metal oxide materials used in capacitors are oxides of Ru, Co, Ni, Mn, Zn, Fe and other elements. Ruthenium oxide, which has excellent redox reversibility, high conductivity, wide electrochemical window, has high energy density, power density and cycle stability. However, the high cost and environmental harmfulness of ruthenium limit its application in commercial supercapacitors.
In recent years, cheap and environment-friendly metal oxide electrode materials have attracted more and more attention from researchers, such as MnO2, NiO, Co3O4 and Fe3O4. Among them, MnO2 has received the most attention due to its relatively low cost, low toxicity, environmental friendliness and high theoretical capacity (1100~1300F). In recent years, with the gradual development of graphene, carbon nanotubes and other materials, the introduction of graphene, carbon nanotubes into transition metal oxides to prepare composite materials has become a research hotspot. Compared with single electrode materials, composite materials have better electrochemical performance.
3.1.3 Conductive polymer electrode materials
Conductive polymers, such as polyaniline (PANi), polypyrrole (PPy), and polythiophene (PTh), are intriguing materials for supercapacitor electrode applications due to their unique properties, including low cost, high conductivity, wide electrochemical window, and high theoretical capacity. These materials store energy through redox reactions, with ions transferring to and from the polymer structure during oxidation and reduction processes. However, their volume expansion and contraction during ion embedding and deintercalation can lead to a decrease in electrochemical performance and cycle stability. Several methods are employed to address this issue:
- Improving Structure and Morphology: Modifying the structure and morphology of conductive polymers, such as creating nanowires, nanorods, and nanotubes, can reduce the volume expansion and contraction during cycling. These nanostructures provide better mechanical stability and minimize the stress on the material.
- Using Carbon Materials as Negative Electrode: The p-type doped state of conductive polymers is more stable than the n-type doped state. To enhance cycle stability, carbon materials can be used as the negative electrode instead of n-type doped polymers. Carbon materials are more stable and maintain their structure during charge and discharge cycles.
- Composite Electrode Preparation: Preparing composite electrodes by combining conductive polymers with other materials, like carbon or metal oxides, can enhance the chain structure, conductivity, mechanical stability, machinability, and stress dispersion. These improvements contribute to better electrochemical stability and overall performance.
These approaches aim to minimize the volume changes in conductive polymer electrode materials during cycling, thus improving the durability and cycle stability of supercapacitors.
3.2 Supercapacitor Electrolyte
Regarding the electrolyte, it plays a crucial role in determining the performance of supercapacitors. There are various types of electrolytes used in supercapacitors:
3.2.1 Water Electrolyte
Water-based electrolytes are among the earliest and most widely used in supercapacitors. They are cost-effective, offer high conductivity, and are easily impregnated into electrode materials. Water-based electrolytes can be categorized into acid (e.g., H2SO4), alkaline (e.g., KOH), and neutral systems. Acid electrolytes offer high ion concentration and low resistance, while alkaline electrolytes also provide high conductivity and low internal resistance. Neutral electrolytes are less corrosive and safer to handle. However, the main drawbacks of water-based electrolytes are their narrow electrochemical window, low oxidation decomposition voltage, low energy density, and poor low-temperature performance.
3.2.2 Organic Electrolyte
Organic electrolytes are another common choice for supercapacitors. They offer a broader electrochemical window compared to water-based electrolytes. However, they are often more expensive and less conductive. Organic electrolytes can be formulated to provide better low-temperature performance, and they are commonly used in commercial supercapacitors.
3.2.3 Ionic Liquid Electrolyte
Ionic liquids are a type of non-aqueous electrolyte that offers a wide electrochemical window, high thermal stability, and low volatility. They are more suitable for high-performance supercapacitors and are often used in research settings. Ionic liquids are, however, more expensive and may have issues with viscosity.
3.2.4 Solid Electrolyte
Solid electrolytes are a promising alternative that can address some of the limitations of liquid electrolytes. They offer improved safety, longer cycle life, and a wider operating temperature range. Solid-state supercapacitors are still an area of active research and development.
The choice of electrolyte depends on the specific requirements of the supercapacitor, including its performance, safety, and cost considerations. Researchers continue to explore and develop new electrolyte materials and formulations to enhance the performance of supercapacitors.
4 Classification of supercapacitor
According to different standards, supercapacitors can be divided into different categories.

Button Supercapacitor

Wound supercapacitor

Winding combination supercapacitor
- Electrode Material:
- Carbon-Based Supercapacitors: These are the most common type and use activated carbon or graphene as the electrode material.
- Pseudocapacitors: These supercapacitors use transition metal oxides or conductive polymers as the electrode material. They offer higher energy density compared to pure carbon-based supercapacitors.
- Electrolyte Type:
- Aqueous Supercapacitors: Use water-based electrolytes, often containing potassium hydroxide (KOH) or sulfuric acid (H2SO4). They tend to have lower energy density but are safer and more environmentally friendly.
- Organic Electrolyte Supercapacitors: Use non-aqueous organic solvents as the electrolyte, which can provide higher energy density but are typically less stable and may require special packaging.
- Construction:
- Symmetric Supercapacitors: These supercapacitors have identical electrodes and are often used in low-voltage, high-power applications.
- Asymmetric Supercapacitors: They use different materials for the positive and negative electrodes to optimize both energy and power density. These are suitable for a wide range of applications, including hybrid electric vehicles.
- Form Factor:
- Button Cell Supercapacitors: Small, cylindrical supercapacitors used in electronic devices.
-
- Coin Cell Supercapacitors: Slightly larger than button cells and often used in backup power applications.
- Prismatic Supercapacitors: Rectangular or pouch-shaped supercapacitors used in larger energy storage applications.
- Wound and Cylindrical Supercapacitors: Used in high-power applications and resemble traditional cylindrical batteries.
- Performance:
- High-Power Supercapacitors: Designed to deliver rapid bursts of energy and are often used in applications like regenerative braking in electric vehicles.
- High-Energy Supercapacitors: Optimized for energy storage and can provide longer discharge times, suitable for applications like renewable energy storage.
- Temperature Range:
- Standard Temperature Supercapacitors: Designed for typical environmental conditions.
- High-Temperature Supercapacitors: Operate in extreme temperature conditions.
- Low-Temperature Supercapacitors: Designed for cold environments.
- Hybrid Supercapacitors:
- Combine the characteristics of batteries and supercapacitors, offering a balance between energy density and power density. They are used in various applications, including electric vehicles and energy storage.
The choice of supercapacitor type depends on the specific requirements of the application, such as energy storage capacity, power delivery, operating conditions, and cost considerations. Different supercapacitor types are used in a wide range of industries, including transportation, renewable energy, consumer electronics, and more.
5 Applications of Supercapacitor
Supercapacitor, with its many advantages, has been widely concerned by people once it came out, and has been successfully applied in many fields. It serves as a backup power supply for electronic products such as memory, computers, timers, etc. It is used as an auxiliary power supply for electric vehicles and hybrid electric vehicles, solar and wind power generation devices, and can also be used in military, aerospace and other fields.
5.1 Applications of supercapacitor in the electronics industry
The super capacitor can be charged in a short time, and can provide relatively large energy, which can be used as a backup power supply for memory, microcomputer, system motherboard, clocks, etc. When the main power supply is interrupted or the system voltage is reduced due to poor contact, the super capacitor can play a backup role to avoid the impact on the instrument caused by sudden power failure. Super capacitors can replace batteries as power supply for small electrical appliances such as electric toys, clocks, cameras, recorders, portable cameras, etc. Super capacitors are also ideal for digital wireless applications. The ultracapacitor can also be used on data recording equipment working in a rather harsh environment, such as counting equipment or package detector.
5.2 Electric vehicle and hybrid vehicle
The requirements of electric vehicles for power supply have attracted worldwide attention to ultracapacitor, a new type of energy storage device. Secondary batteries, including fuel cells, have some limitations in terms of high power output, rapid charging, wide temperature range use, etc. Super capacitors can better meet the power requirements of electric vehicles, especially hybrid electric vehicles when starting, accelerating, and climbing. If it is used together with the power battery, it can act as a large current or energy buffer zone to reduce the damage of large current charging/discharging to the battery and extend the service life of the battery. At the same time, the instantaneous energy can be recovered into the ultracapacitor through the regenerative braking system to improve the energy utilization rate.
5.3 Auxiliary power supply of solar and wind power generation devices
The supercapacitor can be used as the auxiliary power supply of solar or wind power generation devices. It can store the instantaneous large current generated by the power generation device due to the instability of the wind source and light source intensity at a fast speed and release it according to the design requirements, thus greatly increasing the stability of the power grid. In addition, the characteristics of ultracapacitor, such as long life, maintenance free and environmental protection, are also convenient for long-term maintenance free work in the field and become a real green energy. For example, in the daytime, the solar energy provides power and charges the supercapacitor, and in the evening, the ultracapacitor provides power. As the use and maintenance requirements of super capacitors are extremely low, and the service life can reach 10 years, this new type of beacon light can greatly reduce the intensity of daily maintenance work and ensure long-term reliable work.
5.4 Military, aerospace
In addition to the conventional high specific energy battery, the new generation spacecraft must be combined with a super capacitor to form a “compact ultra-high power pulse power supply” during the launch phase. By adjusting the pulse release rate, pulse density and peak release power, the pulsed electric takeoff accelerator, arc jet thruster and other devices can reach any average power level under the pulse state.
As a new type of energy storage element, ultracapacitor has great potential in electric vehicles, electromagnetic weapons and uninterruptible power supply due to its super capacity and high energy storage density. In the practical process, especially in the wider application of pulse power technology, the problems of low working voltage and large internal resistance need to be solved.
The two main applications of supercapacitor are high power pulse application and instantaneous power holding application. High power pulse applications are characterized by large currents flowing instantaneously to the load. Instantaneous power retention applications are characterized by the requirement to continuously provide power to the load. A typical application of instantaneous power retention, which generally lasts for a few seconds or minutes, is to reset the disk drive head when power is lost. Different applications require different parameters of ultracapacitor. High power pulse applications utilize the small internal resistance (R) of supercapacitor, while instantaneous power retention applications utilize the large electrostatic capacity (C) of ultracapacitor
In the future, the research focus of supercapacitor will still be on the development and design of new materials to obtain ideal electrode materials and systems, improve the performance of capacitors, and prepare new power supplies with good performance, low price and convenient use to meet the market demand.
Conclusion
Supercapacitor have come a long way since their historical origins, evolving into versatile energy storage devices with numerous applications. Their unique features, such as rapid charging, long lifespan, and high power density, make them essential in various fields, from electronics to electric vehicles and renewable energy systems. While challenges and limitations remain, ongoing research promises to enhance their performance and expand their role in a sustainable and efficient energy future.




